Yuen Kwok-Yung: Actions needed to prepare for COVID-19 vaccines
Chinese version of this article was published in Ming Pao on 25th December 2020
Written by David Lung, Honorary Assistant Professor; Kelvin Chiu, Honorary Assistant Professor; Siddharth Sridhar, Assistant Clinical Professor; and Yuen Kwok-Yung, Chair Professor, of the Department of Microbiology, Li Ka-shing Faculty of Medicine, HKU.
The article is published under Creative Commons license CC BY-ND 4.0 . Click on link for share conditions.
The objectives of this COVID-19 vaccination program
A high uptake of effective SARS-CoV-2 vaccines is likely to significantly control the COVID-19 epidemic in Hong Kong by accelerating herd immunity against the virus (Ref 1). This will minimize the morbidity, mortality, burden on healthcare and quarantine facilities, and the financial burden on the government. Moreover, normal socioeconomic activities and overseas connections can be restored. Hong Kong should aim to vaccinate the entire population within the shortest possible time frame. As clearly seen in various regions around the world, achieving and sustaining zero COVID-19 cases is very challenging. Border control is not leak-proof because 2.5 to 5% of imported cases have an incubation period of less than 14 days. Many COVID-19 patients may not have symptoms, thereby spreading the virus to those around them unknowingly. Even for those who seek testing, there is a small possibility of COVID-19 patients having false negative test results due to suboptimal sample collection. Compliance to specific counter-measures at critical control points by businesses and the public cannot be fully enforced. Therefore, the SARS-CoV-2 vaccines provide an additional critical layer of protection to sustainably suppress COVID-19 in Hong Kong.
This urgently needed vaccination program cannot be successful without complete transparency to win the support from the general public. The choice of each individual on both the timing and type of vaccines to be administered must be respected.
Acknowledging the unknowns and limitations of the three vaccines
It must be acknowledged that coronavirus vaccines have never before been administered to humans outside study settings. Furthermore, two of the three vaccines bought by Hong Kong are based on new technologies which have never been used in our routine immunization program. Their emergency use must be authorized and carried out according to the best international practice with continuous monitoring of their safety, quality and efficacy after launching of this program.
We know from animal studies that all three vaccines induce sufficient serum neutralizing antibodies which largely protect recipients from symptomatic COVID-19 and complications, but may not necessarily stop asymptomatic virus infection, shedding and transmission in the upper airway. Moreover, we do not know the duration of protection after vaccination and if there will be a booster vaccination is needed months or years after the second dose.
Allergic reactions to residual or stabilizing chemicals inside the vaccines are inevitable. Thus the checking of the history of allergy is mandatory for every consenting recipient.
Furthermore, some rare and serious side effects may not be detected with 10,000 vaccinees in study settings, but may be detected when vaccination is carried out on a large scale involving millions of people. These rare autoreactive inflammatory complications such as transverse myelitis, Guillain barre syndrome, and others usually present within 6 weeks after vaccination. It must be emphasized that these serious side effects are extremely uncommon after usually-administered vaccines.
The vaccines to be available in HKSAR (See table 1)
Table 1: Available COVID19 vaccines in Hong Kong SAR
| Products and company | Tozinameran BNT162b2 (BioNTech) | AZD1222 (Oxford-AstraZeneca) | CoronaVac (Sinovac) |
| Mechanism | modRNA (nucleoside-modified RNA) incooperating 1-methyl-pseudouridine (improved RNA stability) whole spike | Modified chimpanzee adenovirus vector 1 (ChAdOx1) with whole spike protein | Inactivated whole virion SARS-CoV-2 |
| Dosing regimen | 30 ug in 0.3mL intramuscular injection for 2 doses 21 days apart | Standard dose around 5×1010 viral particles (0.5 mL); Low dose around 2.5×1010 viral particles (0.22 or 0.5 mL depending on batch)
Intramuscular injection for 2 doses 28 days apart |
3ug in 0.5mL intramuscular injection for 2 doses 14/28 days |
| Adjuvant | Lipid nanoparticles | Nil | Aluminum hydroxide |
About 18 COVID-19 vaccines have reached phase 3 clinical trials. Many of these candidates have published promising data showing that they are immunogenic in animals & in phase 1/2 human clinical trials. These data have been scrutinized by experts at scientific committees of the HK government’s Centre for Health Protection. The government has taken reference from experts and decided to make a number of advanced purchase agreements to get twice the number of vaccines for our 7.5 million population. These vaccines include the CoronaVac (inactivated whole SARS-CoV-2 virion vaccine of Sinovac), the AZD1222 (Oxford-AstraZeneca modified chimpanzee adenovirus vector 1 with spike of SARS-CoV-2), and the Tozinameran BNT162b2 (mRNA vaccine encoding spike of SARS-CoV-2 from BioNTech/ Pfizer).
Constituents of the vaccines and their mechanism of action (see table 2) (ref. 2,3,4,5)
Table 2: Ingredients and Track Record of different vaccines
| Products and company | Tozinameran BNT162b2 (BioNTech) | AZD1222 (Oxford-AstraZeneca) | CoronaVac (Sinovac) |
| Excipients | lipids (e.g. polyethylene glycol, cholesterol etc.),
Potassium chloride, Monobasic potassium phosphate Sodium chloride, Dibasic sodium phosphate dihydrate, Sucrose etc. |
Information not available | Information not available |
| Immunogens | mRNA
Lipid nanoparticle |
Spike protein
Protein of adenovirus vector Residuals from cell culture |
Spike, Envelope, Matrix, Nucleoprotein
Residuals from cell culture Etc. |
| Track record | First mRNA vaccine used in human | Ebola virus vaccine (cAd3-EBO) | Hepatitis A vaccine,
Polio virus vaccine |
| Theoretical merits | Easy to produce and scale up
Stimulates good neutralizing antibody, Cytotoxic T lymphocyte and cell mediated immunity |
Stimulates good neutralizing antibody, Cytotoxic T lymphocyte and cell mediated immunity | Stimulates good neutralizing antibody |
| Theoretical demerits | Required to be stored in cold temperature (-70 oC) to maintain potency along the transportation chain
|
Optimal dosage of vaccine is still uncertain
|
Tendency to induce Th2 response |
The coronaVac contains all the full antigens of the full virion particle including the surface spike, membrane, envelope, abundant nucleocapsid proteins, RNA genome and aluminium hydroxide adjuvant (Ref 2). The exact list of excipients in the vaccine is yet to be announced, but like all inactivated virus vaccines, the vaccine is likely to contain minute quantities of residual proteins from cell culture ingredients (eg. cell debris), chemicals for inactivation (eg. beta-propiolactone) and stabilization. The injected vaccine proteins are taken up by host dendritic cells and macrophages which present the antigens to the lymphocytes. It is likely that vaccination will stimulate mainly a T helper 2 response with a good neutralizing antibody titre directed towards the Spike protein. There would also be non-neutralizing antibodies generated against other internal viral proteins of SARS-CoV-2.
The AZD1222 contains a non-replicating chimpanzee adenovirus encoding the SARS-CoV-2 spike protein. Despite the purification process, minute quantities of residual cell culture ingredients (cell debris) and chemicals for lysis (such as triton 100, likely remain in the vaccine. AZD1222 cause non replicating infection of host cells by the chimpanzee adenovirus which also express SARS-CoV-2 spike. Both adenovirus proteins and SARS-CoV-2 Spike are then recognized by dendritic cells to trigger a Th1 immune response with neutralizing antibody, cell mediated CD4, and cytotoxic CD8 lymphocyte responses against the adenovirus proteins and the SARS-CoV-2 spike protein. Note that there would be neutralizing and non-neutralizing antibodies produced against the Spike and other adenoviral proteins.
Tozinameran BNT162b2 contains the modified RNA of SARS-CoV-2 spike encapsulated in pegylated lipid nanoparticles (0.1 micron diameter) which facilitate RNA entry into host cells. The spike protein is expressed and presented to host immune cells leading to Th1 immune response with neutralizing antibody, cell mediated CD4 and cytotoxic CD8 lymphocyte response against the SARS-CoV-2 spike protein. The vaccine will contain a minute amount of residual enzyme proteins, double stranded RNA and unused nucleoside triphosphates despite purification but will be free of animal components. Note that the polyethylene glycol polymer for encapsidating the RNA can be a source of allergy in humans.
The immunogenicity of the vaccines in phase 1 or 2 clinical trials (table 3) (Ref. 6,7)
Table 3: Comparison of clinical efficacy of vaccines based on phase III clinical trial data
| Products and company | Tozinameran BNT162b2 (BioNTech) | AZD1222 (Oxford-AstraZeneca) | CoronaVac (Sinovac) |
| Clinical Efficacy | 95.0% (at least 7 days after second dose, N = 17411) | 70.4% (2 dose, regardless of dosage used; at least 14 days after second dose, N = 5807)
90.0% (if low dose for first dose, standard dose for second dose, N = 1367) |
Data from phase III trial still not yet published in international journal |
| Efficacy according to age group | 95.6% (16-55 years old, N = 9897)
93.7% (> 55 years old, N = 7500) 94.7% (> 65 years old, N = 3848) 100.0% (> 75 years old, N = 774) |
Information not available | Information not available |
Direct comparison of the immunogenicity of these three vaccines is difficult as there has never been a head-to-head comparison, but it is obvious that Tozinameran BNT162b2 induced higher neutralizing antibody titre than that of the convalescent asymptomatic patients, As for the induction of cell mediated immunity, the Interferon gamma ELIspot test for specific lymphocyte response suggested that Tozinameran BNT162b2 and AZD1222 are better than CoronaVac.
The clinical efficacy of these vaccines in phase 3 clinical trials
Even though the clinical efficacy of CoronaVac is not yet available in the public domain, it is highly likely that this should be much higher than the WHO requirement of over 50%. As for AZD1222, there is uncertainty about the optimal dosing regimen due to the unintended use of a lower dose in a proportion of the vaccine recipients, which requires further confirmation by further clinical trials. But the preliminary estimated clinical efficacy is 90% for those with a low dose priming and full dose boosting, whereas a full dose of priming followed by a full dose boosting has a lower clinical efficacy of 70.4%. As for Tozinameran BNT162b2, its overall clinical efficacy is 95% with over 90% protection across all age groups from 16 to over 75.
The track record of the technology platforms used by of these vaccines
With regards to the vaccine platform, inactivated whole virion vaccines such as CoronaVac has the best historical track record in terms of clinical efficacy, experience and safety. In fact, our presently used polio, rabies and hepatitis A vaccines are all manufactured by this technology. There are theoretical concerns that this approach may be associated with more of a Th2 immune response after encountering the wild type virus which may lead to lung immunopathology with eosinophilic response although this did not occur in monkey models where the vaccine was highly effective in preventing severe COVID-19. Another theoretical concern is antibody dependent enhancement of infection, which happens especially when the neutralizing antibody titre decreases over time, although this theoretically applies to any vaccine platform.
The adenoviral vector technology (AZD1222) has been used in tackling the Ebola outbreaks in Africa but this has never been used widely in routine immunization program.
The mRNA technology (Tozinameran BNT162b2) has no post-marketing track record both in terms of clinical efficacy and safety.
The safety of these vaccines in phase 3 clinical trials (table 4)
Table 4: Comparison of safety profile of vaccines
| Products and company | Tozinameran BNT162b2 (BioNTech) | AZD1222 (Oxford-AstraZeneca) | CoronaVac (Sinovac) |
| Incidence of side effects | Data based on Vaccines and Related Biological Products Advisory Committee Meeting December 10, 2020
84.1% injection site reactions 62.9% fatigue 55.1% headache 38.3% muscle pain 31.9% chills 23.6% joint pain 14.2% fever |
Data based on phase I/II study with full dose vaccine without booster and without paracetamol
83% injection site tenderness 70% fatigue 68% headache 67% injection site pain 61% malaise 60% muscle ache 56% chills 31% joint pain 25% nausea 25% warmth at injection site 18% fever |
Data based on phase II study
For 0/14 schedule phase II (3ug): 23.3% injection site reactions 5.0% diarrhea 3.3% fatigue 3.3% fever 2.5% muscle pain
For 0/28 schedule phase II (3ug): 10.0% infection site reactions 8.3% fatigue 3.3% fever 2.5% headache 1.7% muscle pain |
| Dangerous side effects | Nil reported related to vaccine (except reports of allergic reaction suspected to be related to PEG in United Kingdom) | 2 cases of transverse myelitis reported in phase III trial (labelled as idiopathic for 1 case, and unrecognized MS in another; among 12021 included for safety analysis, i.e. 0.017%) | Nil reported related to vaccine |
The side effects profiles of all 3 vaccines are mild and acceptable. Besides the rate of fever is lowest for coronaVac (3.3%), AZD1222 (18%), and Tozinameran BNT162b2 (14.2%), coronaVac also has the lowest rate of other symptoms amongst these 3 vaccines.
Two cases of transverse myelitis were reported amongst 7174 AZD1222 vaccine recipients although one was attributed to undiagnosed multiple sclerosis. At this stage, the AZD1222 has not yet been approved widely.
A few cases of type 1 immediate hypersensitivity with skin rash and wheezing was reported in Tozinameran BNT162b2 vaccines during recent vaccination campaigns in UK and US. These were attributed to inevitable exposure to the polyethylene glycol (PEG) in our daily food, drug and topical cosmetics which leads to formation of anti-PEG antibodies.
Quality concerns
To ensure the quality of these vaccines, random sampling of each batch should be rapidly performed to exclude contamination of coronaVac and AZD 1222 from adventitious viruses, replication competent adenovirus, and mycoplasma by next generation sequencing. Electron microscopy can be performed to check the number of spikes, integrity of the inactivated whole virions or the morphology of adenoviral vector vaccine. High performance chromatography and mass spectrometry, protein gel electrophoresis and quantitative PCR with or without reverse transcription should be performed to ensure there is no excess unwanted chemicals or degradation of the viral RNA or target virus proteins. Endotoxin assay and culture test for sterility should also be performed.
How should we launch this vaccination campaign?
The Department of Health has all the regulatory power to authorize the emergency use of these 3 vaccines after going through due committees and legislative procedures. Vaccination centers, hospitals and clinics with the necessary cold chain and storage, resuscitation facilities, and medical personnel should be the sites where these vaccines should be administered.
Information technology system should be in place to monitor for safety, efficacy and quality of these vaccines, and the health status of the vaccinees identified by their ID card number during and after the vaccination campaign. Regular batch to batch monitoring of the chemical and microbiological constituents of these vaccines is important to give confidence the public. Any incidents must be immediately conveyed to the general public.
Public education campaign should be launched with all the information available to ensure 100% transparency, and that the vaccination is completely voluntary and free of charge. The public must be educated to understand that illness and deaths occur daily with or without vaccination. Illness or deaths soon after vaccination may not have any causative relationship. There are 15 to 20 Bell’s palsy per 100,000 population per year compared with the extrapolated 11 cases per 100,000 vaccinees (4 out of 38,000 vaccinees in the trial).
CoronaVac appears to be the safest vaccine with minimal side effects and should be well tolerated by elderlies and those with comorbidities. However, due to the relatively lower neutralizing antibody titre and cell mediated immune response, the duration of protection of coronaVac could be shorter. But this could be compensated by future booster vaccination if the serum antibody levels wane with time.
At this stage in time, the available phase 2 and 3 clinical trial data showed that Tozinameran BNT162b2 appears to have proven clinical efficacy and reasonable safety. The content in this vaccine is relatively simple and chemically homogeneous which can be checked easily by laboratory assays.
Thus, Tozinameran BNT162b2 and coronaVac should be lined up as the first-choice vaccines used in HKSAR. Healthcare workers, elderlies, patients with chronic underlying disease, and those at border control and high risk occupation should come first to receive Tozinameran BNT162b2 or coronaVac. They should be given the choice to choose the type of vaccine.
Though the two cases of transverse myelitis could be just bad luck, it is prudent to wait for a longer time till a more proper phase 3 clinical trial of AZD1222 is performed as there is still confusion over the optimal dosing regimen.
We do not have the scientific data on the effect of using 2 different types of SARS-CoV-2 vaccines in one individual. In order not to confuse the effects of different vaccines, it is prudent that no recipient should receive any other vaccines within 6 weeks before and after receiving SARS-CoV-2 vaccination.
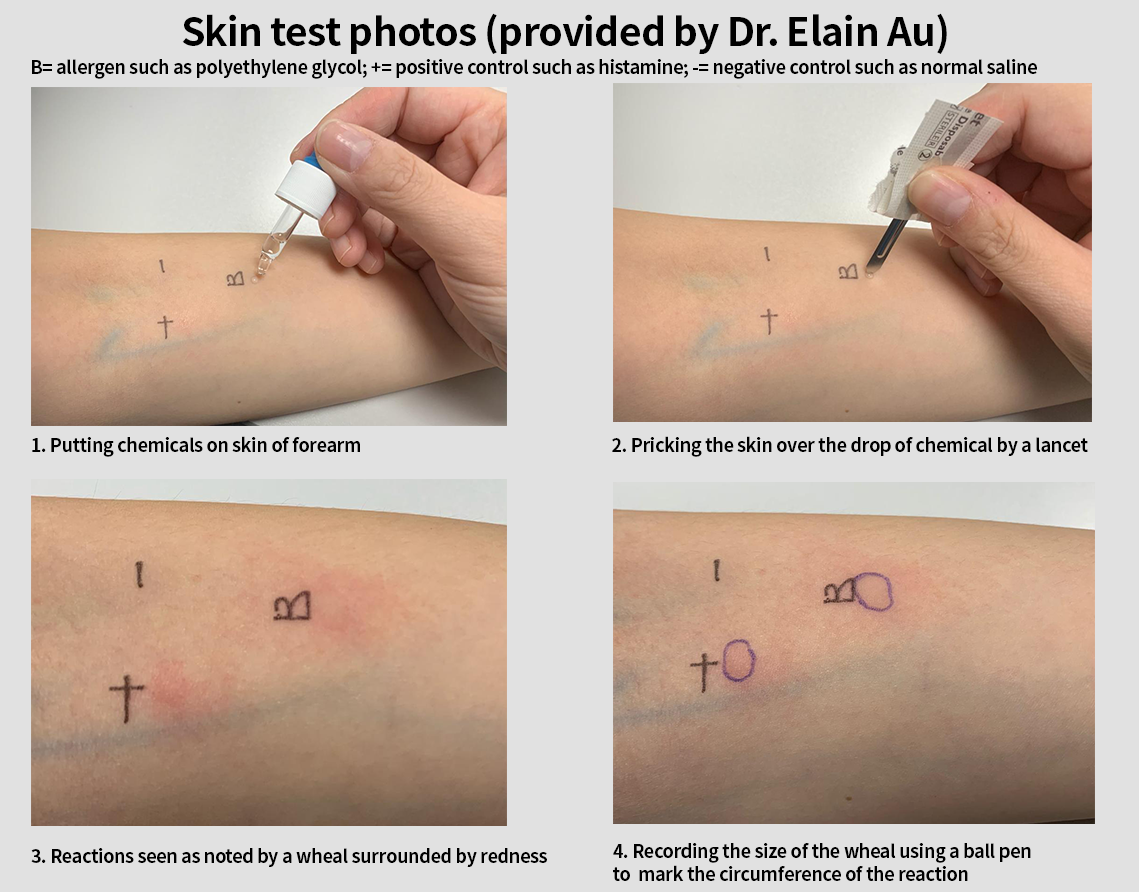
The history of allergy should always be checked to ensure that those with allergy to residual chemicals in coronaVac and AZD1222 (such as penicillin, streptomycin, bovine serum albumin) should not be given these vaccines and be considered for Tozinameran BNT162b2. Note that the incidence of type 1 hypersensitivity to penicillin is 0.02% to 0.04%. Those with history of allergy to PEG should be given coronaVac or AZD1222. Clinical immunologists with experience in doing skin test should help those with uncertain history of allergy before deciding on the type of vaccine used (see skin test photos, Ref. 8).
Reference list
- Fontanet, A., & Cauchemez, S. (2020). COVID-19 herd immunity: where are we?. Nature reviews. Immunology, 20(10), 583–584. https://doi.org/10.1038/s41577-020-00451-5
- Gao Q, Bao L, Mao H, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77-81. doi:10.1126/science.abc1932
- Vaccines and Related Biological Products Advisory Committee Meeting. December 10, 2020. FDA Briefing Document. Pfizer-BioNTech COVID-19 Vaccine. bit.ly/3mN7Lb4 (Accessed on 21 Dec 2020)
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine (published online ahead of print, 2020 Dec 10). N Engl J Med. 2020; 10.1056/NEJMoa2034577. doi:10.1056/NEJMoa2034577
- van Doremalen N, Lambe T, Spencer A, et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586(7830):578-582. doi:10.1038/s41586-020-2608-y
- Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586(7830):589-593. doi:10.1038/s41586-020-2639-4
- Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK (published online ahead of print, 2020 Dec 8). Lancet. 2020; S0140-6736(20)32661-1. doi:10.1016/S0140-6736(20)32661-1
- Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial [published correction appears in Lancet. 2020 Aug 15;396(10249):466] [published correction appears in Lancet. 2020 Dec 12;396(10266):1884]. Lancet. 2020;396(10249):467-478. doi:10.1016/S0140-6736(20)31604-4
- Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial [published online ahead of print, 2020 Nov 17]. Lancet Infect Dis. 2020;S1473-3099(20)30843-4. doi:10.1016/S1473-3099(20)30843-4
- Sellaturay P, Nasser S, Ewan P. Polyethylene Glycol-Induced Systemic Allergic Reactions (Anaphylaxis) [published online ahead of print, 2020 Oct 1]. J Allergy Clin Immunol Pract. 2020;S2213-2198(20)31007-2. doi:10.1016/j.jaip.2020.09.029



