Research
[The Lancet] Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial

The Lancet, May 8 2020
Ivan Fan-Ngai Hung,Kwok-Cheung Lung,Eugene Yuk-Keung Tso,Raymond Liu,Tom Wai-Hin Chung,Man-Yee Chu,Yuk-Yung Ng,Jenny Lo,Jacky Chan,Anthony Raymond Tam,Hoi-Ping Shum,Veronica Chan,Alan Ka-Lun Wu,Kit-Man Sin,Wai-Shing Leung,Wai-Lam Law et al.
Highlights:
- Between Feb 10 and March 20, 2020, 127 patients were recruited and randomly assigned to either the triple combination lopinavir–ritonavir, ribavirin, and interferon beta-1b group or the control group (lopinavir–ritonavir only), in the ratio of 2:1, by simple randomisation with no stratification.
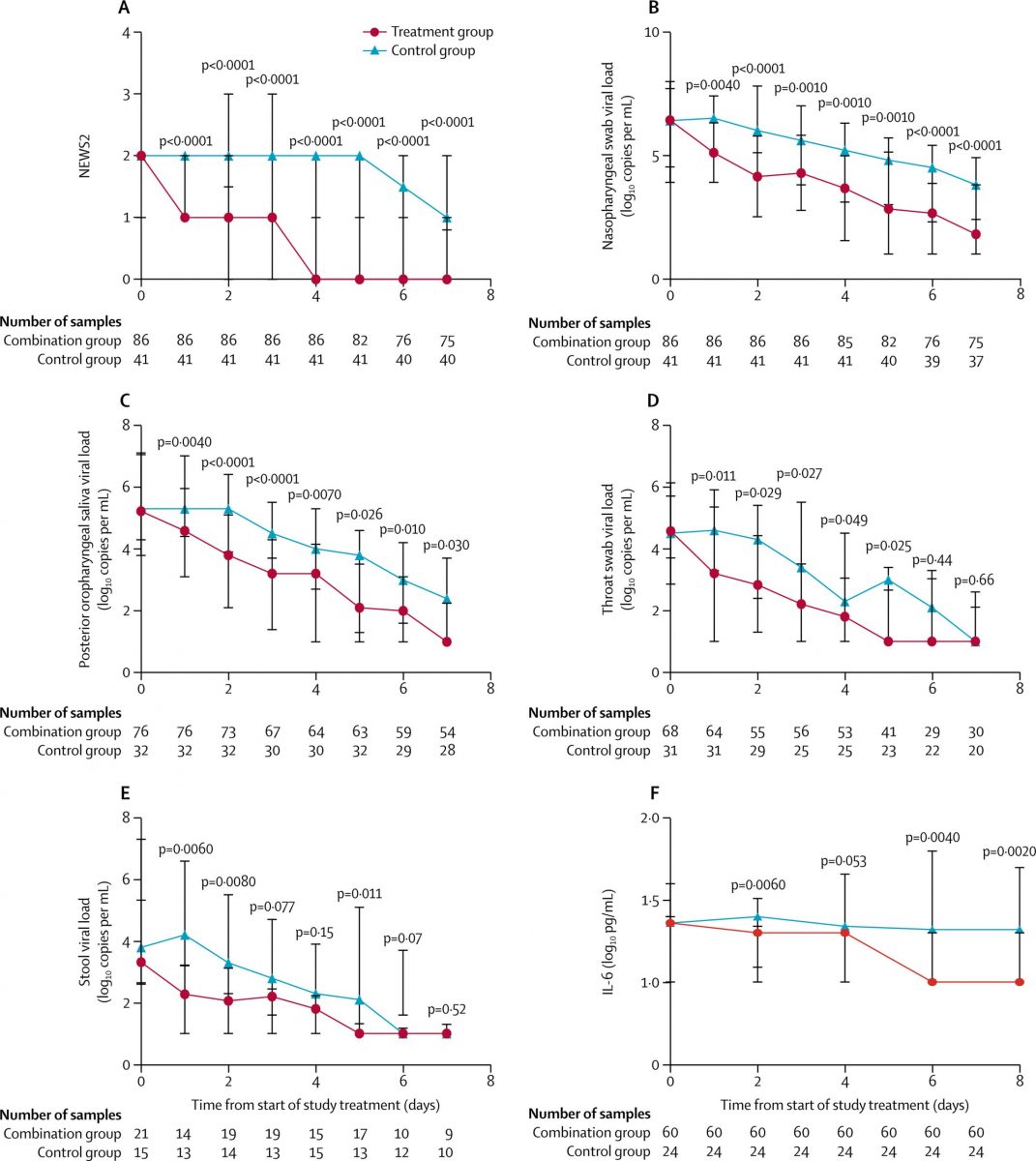
- For the primary endpoint of time from start of study treatment to negative nasopharyngeal swab, the combination group had a significantly shorter median time (7 days [IQR 5–11]) than the control group.
- Clinical improvement was significantly better in the combination group, with a significantly shorter time to complete alleviation of symptoms
- For the virological outcome, the combination treatment was associated with significantly shorter time to negative viral load in all specimens when assessed individually (nasopharyngeal swab, posterior oropharyngeal saliva, throat swab, and stool samples) as well as in all specimens combined
Read full text
Related news coverage:
- 東方日報:雞尾酒療法助減症狀 港大研究:加快出院
- 香港01:港大袁國勇研究證病人用雞尾酒療法 早5.5日離院
- ToPick:【新冠肺炎】藥物治療新曙光 袁國勇團隊發現混合療法有助抑制患者病毒量及改善徵狀

![Photo of [Nature Microbiology] Metallodrug ranitidine bismuth citrate suppresses SARS-CoV-2 replication and relieves virus-associated pneumonia in Syrian hamsters](https://fightcovid19.hku.hk/content/uploads/2020/10/Image-2-390x220.jpg)
![Photo of [Cell Reports Medicine] Oral SARS-CoV-2 inoculation establishes subclinical respiratory infection with virus shedding in golden Syrian hamsters](https://fightcovid19.hku.hk/content/uploads/2020/10/fx1_lrg-e1601870075235-390x220.jpg)